Study Background
Oral agents for chemotherapy are becoming an increasingly common modality for treatment
Patients receiving these treatments experience variable adherence, high symptom burden, yet have limited contacted with their providers compared to patients receiving chemotherapy infusions.
Twelve (12) NCORP community oncology sites, both rural and urban, will be recruited and cluster randomized. Community sites will participate for two years and will recruit 43 patients receiving oral chemotherapy.

As of November 2024, there are 12 approved recruitment sites. Congratulations to the following:
Upstate Carolina NCORP/AnMed Health Cancer Center
CROWN NCORP/Aspirus Regional Cancer Center
Carle Cancer Center NCORP
Ozarks NCORP/Central Care Cancer Center
Ozarks NCORP AF/Lake Regional Health System
Georgia CaRes MU NCORP
Gulf South NCORP/LSU University Medical Center
NCORP of the Carolinas/Prisma Health Cancer Institute
Puerto Rico MU NCORP
CommonSpirit NCORP/Cancer Center at St Joseph's
Cook County MU NCORP/Stroger Hospital
New Mexico NCORP/UNM Comprehensive Cancer Center

Study Design
CC012CD is a cluster-randomized trial where twelve eligible NCORP community sites will be recruited. Six (6) practices will be randomized to the ATSM+TIPC intervention, and six (6) practices will be randomized to an active control (IVR only).
Practices will be asked to identify at minimum one (1) staff members who will be consented to participate in the study as clinic personnel. A total of 43 participants will be enrolled at each community site. For patients to be eligible to participate, they must be initiating treatment with an oral chemotherapy agent.
Intervention: ATSM+TIPC
Participants at these community sites will receive Interactive Voice Response (IVR) telephone symptom monitoring plus referral to self-management strategies provided via a printed handbook (ATSM). Any participants who report unresolved psychological distress in the first four (4) weeks will also receive Telephone Interpersonal Counselling (TIPC) for 8 weeks. Symptom reports from the IVR system will be sent to clinic personnel.
Active Control: IVR only
Participants at these community sites will receive Interactive Voice Response (IVR) telephone symptom monitoring only for 12 weeks. Symptom reports from the ATSM system will be sent to clinic personnel.

Community Site Work Flow
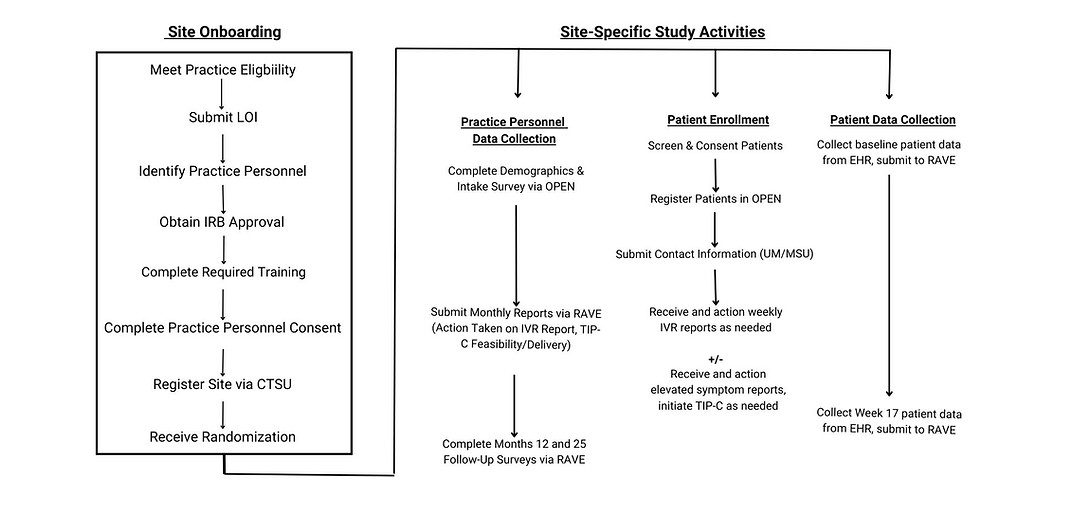
Community Site Eligibility and Participation
If you are interested in opening this trial at your NCORP community site, please see all study documents in CTSU. Contact us to learn more!
Site Training Materials
Winter NRG 2024 Site Training
Summer NRG 2024 Updated Site Training
Monthly Study Updates
September 2024 Study Update
October 2024 Study Update
November 2024 Study Update
December 2024 Study Update
February 2025 Study Update
March 2025 Study Update
April 2025 Study Update
May 2025 Study Update
June 2025 Study Update
July 2025 Study Update
August 2025 Study Update
September 2025 Study Update
October 2025 Study Update
November 2025 Study Update
Other Resources
Study Chairs and Team

Alla Sikorskii, PhD
Study Chair, PI
Michigan State University

Tracy Crane, PhD, RDN
Study Chair, PI
University of Miami

Terry Badger, PhD, RN
Study Chair, PI
University of Arizona

Stephanie Pugh, PhD, Statistician, NRG Stats and Data Management

Vamsi Vasireddy, DO, NCORP Community Chair, Carle Cancer Institute

Melyssa Foust, MSN, RN,
Nursing Co-Chair, Upstate Carolina NCORP

Jamillah Gross-Caldwell,
Study Coordinator - Michigan State University

Grey Freylersythe,
Study Coordinator - University of Miami
Ready to Learn More?
Contact Us!
SYMON@miami.edu
(305) 243-9832





